Accumbal Dopamine and Acetylcholine Dynamics during Psychostimulant Sensitization
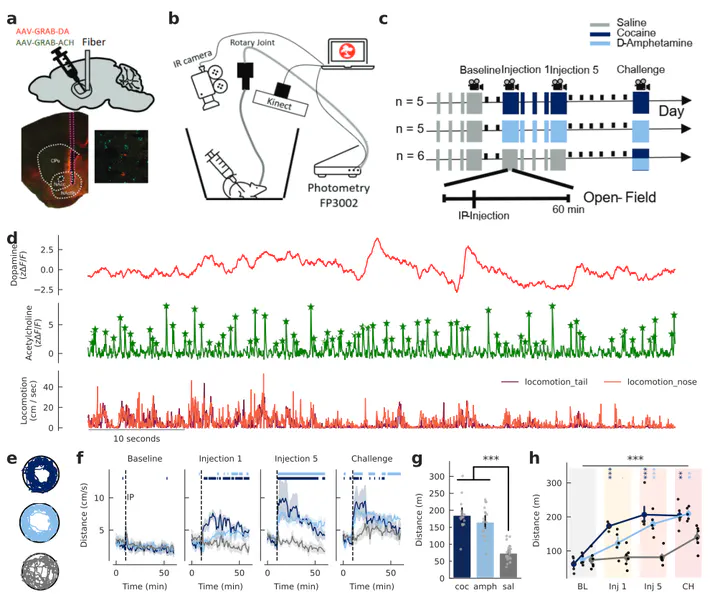 Experimental Setup and Data Recording.
Experimental Setup and Data Recording.Abstract
Behavioral sensitization to repeated psychostimulant exposure is believed to contribute to the development of addiction. Nucleus accumbens (NAcc) dopamine (DA) is known to be a key substrate in sensitization, though recent work suggests that striatal acetylcholine (ACh) may also play a critical role. However, underlying ACh changes and their relationship to DA signaling have not been characterized. Here, we used dual-color fiber photometry to simultaneously measure DA and ACh in the NAcc shell of mice across repeated injections of cocaine or amphetamine. Repeated exposure progressively elevated locomotor activity and increased slow extracellular DA while attenuating transient DA release. Psychostimulants reduced phasic ACh transient amplitude and frequency, an effect that sensitized with repeated injections. However, the temporal coupling of DA and ACh remained unchanged. To determine whether D2 receptors (D2Rs) on cholinergic interneurons (CINs) drive this effect, we generated CIN-selective D2R knockout (KO) mice. Surprisingly, KOs continued to show an acute decrease in ACh and intact DA-ACh correlations after psychostimulant administration. However, they failed to exhibit sensitization of either DA or ACh in response to repeated psychostimulant administration. Despite this lack of sensitization in underlying neuromodulator signaling, the KO mice nevertheless exhibited behavioral sensitization, though at a slower rate than wild-type. These findings suggest that neural sensitization to psychostimulants is dependent on D2R expressed on CINs, but that behavioral sensitization is not dependent on sensitization of these underlying signals.
Type
Publication
BioRxiv Preprint